- TOP
- List of reports
- Confirmation of the Safety of Raw Materials (November 2008)
Confirmation of the Safety of Raw Materials (November 2008)
【Scientific information】
Research and Development Department, Sun Chlorella Corporation
Confirmation of the Safety of Raw Materials (November 2008)
The importance of ensuring the safety of foods has been argued about actively since many years ago. Recently, scandals and events related to foods and the resulting hazards have been reported by mass media almost every day, and consumers have been becoming more interested in food safety. In September 2008, the Law on Installation of Consumer Affairs Agency was proposed to the Diet for the purpose of improving the safety of foods, including improvement in food labeling. This agency is going to be organized in fiscal 2009.
The awareness of people about food safety is now elevating not only in Japan but also in foreign countries such as the USA. The Food and Drug Administration (FDA), USA, made public a plan to open its first overseas office in China by the end of 2008. In each country, the regulations on imported foods have been intensified year after year.
In Japan, Chlorella and Acanthopanax senticosus Harms (hereinafter simply called "Acanthopanax") have been eaten for many years, and their safety has been recognized sufficiently. However, following the changes in social circumstances mentioned above, it is now required to verify the safety of raw materials scientifically and to disclose the results of such verification. For this reason, we recently checked the raw materials used for our products scientifically. In this check, safety of Chlorella powder products and Acanthopanax extracts was endorsed scientifically.
In February 2006, the Ministry of Health, Labour and Welfare announced that one of the "Agaricus" products on the markets was shown to stimulate carcinogenesis. Agaricus subrufescens (Iwade 101 strain), which has been used as a raw material at our company, has been shown to have no problem in the safety and toxicity studies (genotoxicity test, etc.) conducted at the National Institute of Health Sciences, and the data from these studies have been published on our website. Recently, safety of C.G.F. Combined Agaricus subrufescens (a mixture of Agaricus subrufescens and C.G.F.) was endorsed scientifically, as shown below.
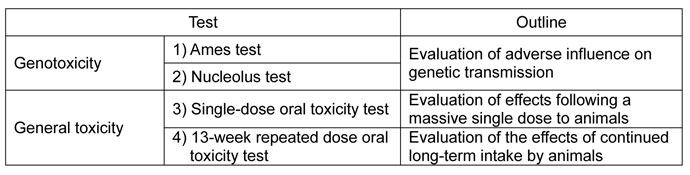
1. Chlorella powder products, 2. Acanthopanax extracts
- Tests
- The tests were conducted in accordance with the strict standards applicable to development of pharmaceutical products.
- Results
- In the tests listed above, safety of Chlorella powder products and Acanthopanax extracts was confirmed.
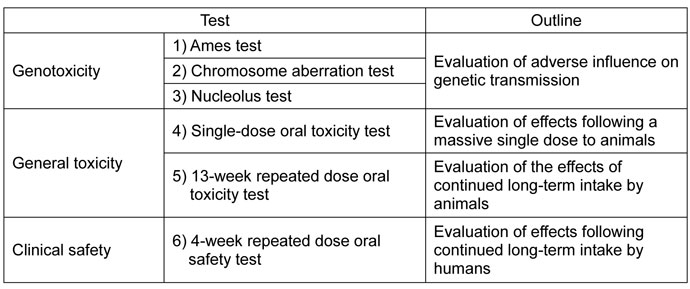
3. C.G. F. Combined Agaricus subrufescens
- Tests
- The tests were conducted in accordance with the strict standards applicable to development of pharmaceutical products.
- Results
- In the tests listed above, safety of C.G. F. Combined Agaricus subrufescens was confirmed.
Relevant information
*Safety of Agaricus subrufescens used as raw material





